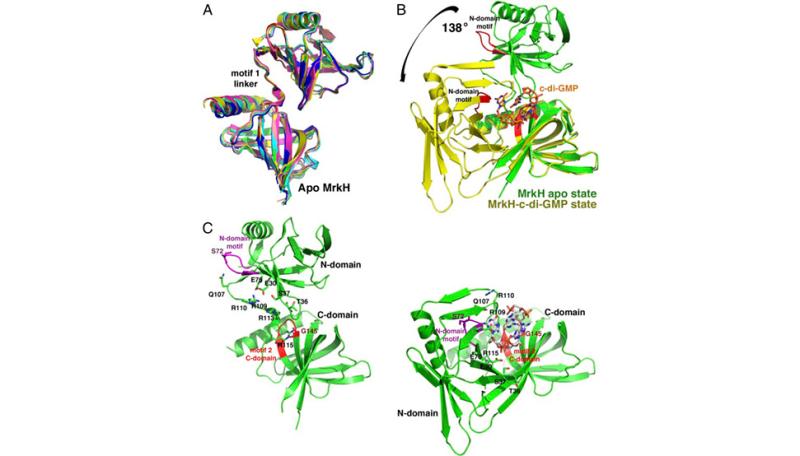
Klebsiella pneumonia is an important cause of refractory nosocomial infections, the infectivity of which is largely due to its ability to form biofilms on biomedical devices. Mannose-resistant (Mrk) hemagglutinins are critical for K. pneumonia biofilm development and the expression of the genes encoding these proteins are activated by a c-di-GMP regulated transcription factor, MrkH. The findings show that MrkH consists of two domains, both of which have PilZ-like folds. Comparison of apo and c-di-GMP-bound MrkH structures reveal a large 138° interdomain rotation induced by binding an intercalated c-di-GMP dimer. C-di-GMP interacts with PilZ C-domain motifs 1 and 2 (RXXXR and D/NxSxxG) and a newly described c-di-GMP binding motif in the N-domain. Unexpectedly, the c-di-GMP binding motifs also stabilize the open state conformation of the apo form via contacts from the PilZ motif 1 to residues in both C-domain c-di-GMP binding motifs. Utilization of the same regions in apo structure stabilization and c-di-GMP interaction allows binding of the second messenger to act as a conformational switch. Data indicate that the domain reorientation caused by c-di-GMP complexation with MrkH activates it to bind DNA. PilZ domains are known signaling modules but MrkH is the first PilZ containing protein to function in DNA binding. MrkH shows no homology to any human protein. Hence, these combined data set the stage for the rational development of novel antimicrobials that target biofilm formation by Klebsiella pneumonia. Read more