In a recent paper published online by Molecular Cell, research from the Schumacher and Brennan laboratories unveils that c-di-GMP controls Streptomyces development and the structural mechanism behind this control.
c-di-GMP and Streptomyces
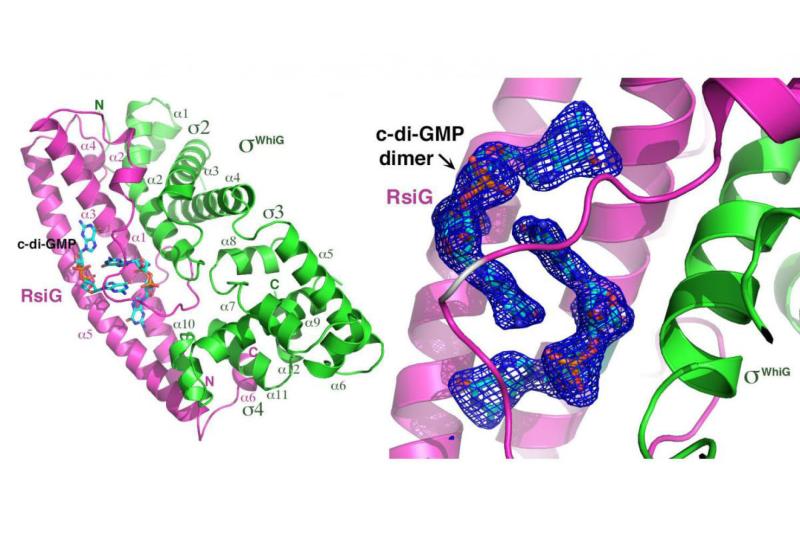
Bacteria of the genus Streptomyces serve as our primary source of antibiotics, which they produce concomitant with a complex developmental life cycle that involves two main steps: first the transition from vegetative growth to the erection of aerial hyphae, and second, the production of spores. Previously we showed that the signalling molecule c-di-GMP binds BldD, a master repressor of sporulation, to control the initiation of development and our crystal structure of the BldD complex with c-di-GMP revealed a novel binding mechanism of this modified nucleotide to allow BldD to function properly. In a new study published in Molecular Cell, Schumacher (the lead author at Duke) and Brennan, in collaboration with the Buttner laboratory at the John Innes Centre, showed that c-di-GMP also intervenes in the sporulation step by arming a novel anti-σ (RsiG) to bind and sequester a sporulation-specific σ factor (σWhiG). This was revealed in the crystal structure of the RsiG-(c-di-GMP)2-σWhiG complex, which showed an unusual partially intercalated c-di-GMP dimer bound at the RsiG-σWhiG interface. The structure also revealed that RsiG binds c-di-GMP in the absence of σWhiG by employing a newly described c-di-GMP binding motif, E(X)3S(X)2R(X)3Q(X)3D, which is repeated on each helix of a coiled-coil. Our further studies demonstrated c-di-GMP is essential for RsiG to inhibit the function of σWhiG. Thus, these findings revealed a newly described control mechanism for σ-anti-σ complex formation and critically, have established c-di-GMP as the central integrator of Streptomyces development and hence, antibiotic production.